Abstract
Background
AML may be initiated by cytogenetic alterations or mutations in genes encoding epigenetic regulators. Epigenetic dysregulation influences the transformation of hematopoietic stem cells or their downstream progenitors into self-renewing leukemic stem cells (LSCs), which contribute to AML pathogenesis and tumor growth. Residual chemoresistant LSCs are implicated in relapsed and/or refractory (R/R) AML, a life-threatening disease with limited treatment options.
The epigenetic eraser LSD1 demethylates histone lysine residues to alter gene expression, is essential for hematopoiesis, and is often overexpressed in LSCs in AML. CC-90011 is a potent, selective, and reversible oral inhibitor of LSD1 that has shown antitumor effects in solid-tumor and AML cell-line models. CC-90011 monotherapy had a favorable safety profile and showed evidence of antitumor activity in patients with advanced solid tumors and R/R non-Hodgkin lymphoma (Hollebecque et al. ESMO TAT 2021. Abstract 7O). CC-90011 combined with etoposide plus carboplatin or cisplatin was well tolerated in patients with extensive-stage small cell lung cancer (Ponce at al. ELCC 2021. Abstract 50P).
VEN plus AZA has emerged as standard therapy for elderly patients with AML. Adding CC-90011 to VEN and AZA may inhibit the aberrant LSD1 activity associated with AML pathogenesis and LSC propagation, increase sensitization to VEN and AZA, and produce deeper and more durable responses than VEN plus AZA alone.
Study Design and Methods
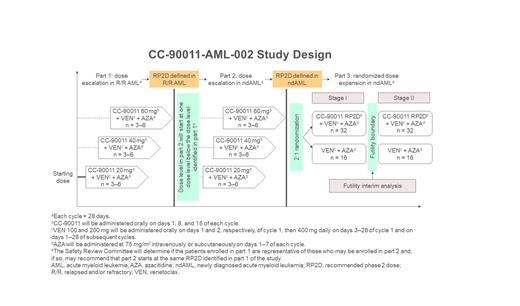
CC-90011-AML-002 (NCT04748848) is a phase 1/2, open-label, multicenter study to evaluate the safety, tolerability, and preliminary efficacy of CC-90011 plus concurrent VEN and AZA in adult patients with R/R AML or in patients with newly diagnosed AML (ndAML) who are ≥ 75 years of age or are 18-74 years of age and ineligible for intensive induction chemotherapy. The study has 2 dose-escalation parts in patients with R/R AML (part 1) or ndAML (part 2), and a randomized dose-expansion part in patients with ndAML (part 3). Part 3 will use a 2:1 randomized design with Bayesian informative prior to calculate the posterior probability that the complete remission (CR) rate in the treatment arm is higher than in the control arm. Enrolled patients must have a projected life expectancy of ≥ 12 weeks, ECOG performance status of 0-2, white blood cell count ≤ 25 × 10 9/L, and adequate organ function. Patients will be excluded if they are candidates for FLT3 inhibitor therapy or have suspected or proven acute promyelocytic leukemia, favorable-risk cytogenetics, or central nervous system involvement.
In parts 1 and 2, patients will receive CC-90011 20, 40, or 60 mg plus VEN and AZA (3-6 patients per treatment arm). In part 3, patients will receive VEN plus AZA with or without CC-90011 administered at the recommended phase 2 dose (RP2D) determined in part 2 (approximately 64 and 32 patients, respectively), with an interim analysis for futility once 50% of patients have been randomized and completed 3 treatment cycles. In all parts, CC-90011 will be administered orally on days 1, 8, and 15 of each 28-day cycle, AZA 75 mg/m 2 will be administered intravenously or subcutaneously on days 1-7 of each cycle, and oral VEN 400 mg will be administered on days 1-28 of each cycle, with a dose ramp-up on days 1 and 2 of cycle 1. VEN will be given ≥ 6 hours after CC-90011 to minimize drug-drug interactions. For clinical outcome evaluation, patients should be treated for ≥ 3 cycles but can discontinue sooner due to disease progression, unacceptable adverse events, intercurrent illness, or investigator's decision.
Primary objectives are to evaluate the safety and tolerability of CC-90011 plus VEN and AZA, and to determine the maximum tolerated dose and/or RP2D of CC-90011. Secondary objectives are to assess the preliminary efficacy of CC-90011 plus VEN and AZA in parts 1-3, and to evaluate the minimal residual disease (MRD) response and conversion rates by multicolor flow cytometry and/or next-generation sequencing in parts 2 and 3. Preliminary efficacy will be determined using CR rate, rate of CR with partial or incomplete hematologic recovery, overall response rate, and duration of response in parts 1-3, and event-free and overall survival in part 3. Because CC-90011 is expected to target LSCs, its addition to VEN plus AZA is predicted to increase the depth and durability of response by MRD evaluation compared with control, rather than increase remission rates.
DiNardo: AbbVie: Consultancy, Research Funding; Novartis: Honoraria; Foghorn: Honoraria, Research Funding; Takeda: Honoraria; ImmuneOnc: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Forma: Honoraria, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Agios/Servier: Consultancy, Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Borthakur: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ryvu: Research Funding; GSK: Consultancy; Astex: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees; University of Texas MD Anderson Cancer Center: Current Employment; Protagonist: Consultancy; ArgenX: Membership on an entity's Board of Directors or advisory committees. Erba: AbbVie Inc; Agios Pharmaceuticals Inc; ALX Oncology; Amgen Inc; Daiichi Sankyo Inc; FORMA Therapeutics; Forty Seven Inc; Gilead Sciences Inc; GlycoMimetics Inc; ImmunoGen Inc; Jazz Pharmaceuticals Inc; MacroGenics Inc; Novartis; PTC Therapeutics: Research Funding; AbbVie Inc; Agios Pharmaceuticals Inc; Bristol Myers Squibb; Celgene, a Bristol Myers Squibb company; Incyte Corporation; Jazz Pharmaceuticals Inc; Novartis: Speakers Bureau; AbbVie Inc; Agios Pharmaceuticals Inc; Astellas; Bristol Myers Squibb; Celgene, a Bristol Myers Squibb company; Daiichi Sankyo Inc; Genentech, a member of the Roche Group; GlycoMimetics Inc; Incyte Corporation; Jazz Pharmaceuticals Inc; Kura Oncology; Nov: Other: Advisory Committee; AbbVie Inc: Other: Independent review committee. Mawad: Abbvie: Speakers Bureau. Kremyanskaya: Protagonist Therapeutics: Consultancy, Research Funding; Incyte: Research Funding; Constellation: Research Funding; Astellas: Research Funding; Bristol Myers Squibb: Research Funding; Chimerix: Research Funding; Astex: Research Funding. Blachly: AstraZeneca: Consultancy, Honoraria; KITE: Consultancy, Honoraria; INNATE: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Carraway: Celgene, a Bristol Myers Squibb company: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Other: Independent review committee; AbbVie: Other: Independent review committee; Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astex: Other: Independent review committee; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Youn: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Garzon: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Lopes de Menezes: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Martin-Regueira: Bristol Myers Squibb: Current Employment, Current holder of individual stocks in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Beach: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Watts: Genentech: Consultancy; Bristol Myers Squibb: Consultancy; Takeda: Consultancy, Research Funding; Rafael Pharmaceuticals: Consultancy; Jazz Pharmaceuticals: Consultancy; Aptevo Therapeutices: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal